Aims & scope Multiple Sclerosis is an area of ever expanding research and escalating blogger.comle Sclerosis and Related Disorders is a wide ranging international journal supported by key researchers from all neuroscience domains that focus on MS and associated disease of the central nervous blogger.com primary aim of this new journal is the rapid Casanova et al. P – Rituximab – Ocrelizumab comparison in real-world practice Introduction: Several anti-CD20 drugs have demonstrated their efficacy in multiple sclerosis (MS) but there is an ongoing debate about the differences of these blogger.comive and aims: To compare the effectiveness and safety profile of rituximab (RTX Oct 26, · Additional research is needed to better understand who might benefit from this procedure and how it compares to the benefits of powerful immune-modulating therapies now available. Read a summary of the results or the paper in JAMA Neurology • In February , results were published from a multi-center, 5-year trial called the HALT MS Study
Multiple Sclerosis
Then the stored hematopoietic stem cells are reintroduced to multiple sclerosis research paper body. The new stem cells migrate to the bone marrow and over time reconstitute the immune system. The medications and procedures used in HSCT are already approved by the FDA. Publication of the outcomes from well controlled clinical studies of HSCT therapy will encourage greater acceptance and use by the medical community.
The goal of HSCT is to reset the immune system and stop the inflammation that contributes to active relapsing MS. The National Medical Advisory Committee of the National MS Society has written an article reviewing evidence related to the optimal use of autologous HSCT for the treatment of specific types of relapsing multiple sclerosis. There is growing evidence that aHSCT is not for everyone with MS but may be highly effective for people with relapsing MS who meet very specific characteristics.
While the general multiple sclerosis research paper is the same, there are different treatment protocols that vary depending on the medical center and doctors performing the procedure. In general, these are some of the steps involved: A person undergoing autologous HSCT to treat MS is given some form of chemotherapy, usually by infusion in the vein, for days, and also another type of treatment for up to 10 days to stimulate the multiple sclerosis research paper of bone marrow stem cells and promote their release into the blood.
Then some blood is drawn from a vein and the stem cells in the blood are stored for later use. Then the individual is usually hospitalized, and given a powerful mix of chemotherapies for several days to kill or suppress immune cells throughout the body. The stored stem cells are then infused into the bloodstream through a vein.
The individual is usually given medicines such as antibiotics to help combat possible infection. The person remains in the hospital for an additional period of time while the immune system begins to rebuild itself. Sometimes individuals are discharged from the hospital in two to four weeks, multiple sclerosis research paper, but this can be longer. The immune system gradually rebuilds itself within 3 to 6 months.
Read more frequently asked questions about HSCT and MS. Additional research is focusing on figuring out who might benefit from this procedure and how to reduce its risks. HSCT is being investigated in Canada, the United States, Europe and elsewhere. This study is now recruiting participants nationwide. The Society has been engaged with the team conducting the trial and is helping with recruitment. Multiple sclerosis research paper trial is testing treatment with HSCT compared with alemtuzumab in people with active relapsing-remitting MS.
Read more about this trial on clinicaltrials. Richard Burt to determine the optimal protocol for safety and benefit is ongoing and no longer recruiting participants. According to Northwestern, Dr. Burt will be going on a planned research sabbatical at the end of Thirteen people who did not respond to conventional therapies for highly active relapsing MS underwent autologous HSCT and completed 24 months of follow up.
Disability progression was measured by the EDSS scale. Two people had one relapse during the first year and three people had one relapse during the second year. EDSS improved in 11 participants Information processing speed and verbal learning improved multiple sclerosis research paper at 12 months, multiple sclerosis research paper. No transplant-related deaths occurred. Fever was the most common side effect. Most showed signs of clinical or MRI inflammatory activity in the year prior to the procedure.
No relapses or inflammatory activity occurred on MRI scans after treatment. These results suggest that HSCT might be appropriate in a subgroup of people with SPMS that have significant inflammatory activity as measured by MRI. Further study in larger numbers are needed to understand who among those with secondary progressive MS might benefit from HSCT. Read a summary of the findings or an abstract in The MS Journal.
Burt, MD MD going on research sabbatical late from Northwestern University, Chicago, IL published multiple sclerosis research paper of the first randomized, control trial of bone marrow stem cell transplant HSCT in people with aggressive relapsing-remitting MS, multiple sclerosis research paper. They enrolled people whose MS was not controlled by available disease-modifying therapies.
Half received immunosuppressant therapy followed by hematopoietic blood cell-producing stem transplant. The other half were switched to a different disease-modifying therapy. Significantly fewer people experienced MS progression in the group that underwent HSCT, compared with the group who were switched to a different MS disease-modifying therapy.
There were no deaths or life-threatening adverse events in either group. The investigators consider this study to be preliminary and recommend that further research is needed to confirm these findings and to determine longer-term outcomes and safety. Read the summary or read the abstract in JAMA.
John Moore, David Ma St. This trial enrolled 35 people with relapsing-remitting MS or secondary progressive MS whose disease had not responded well to disease-modifying medications.
There was no control group or blinding; all participants underwent the HSCT procedure. The team reported on results after following participants from 12 to 66 months after transplantation. EFS was better in those who had relapsing MS. Of 8 who experienced MS progression after transplantation, 2 had relapsing-remitting MS and 6 had secondary progressive MS.
Twelve of thirteen whose disability scores improved after transplantation had relapsing-remitting MS. At this center, which has a long experience multiple sclerosis research paper bone marrow transplants, there were no transplant-related deaths. Read a summary or read the abstract in the JNNP. They found that overall, the procedure showed a significant benefit against disease activity and progression. The pooled results showed an overall transplant-related mortality rate of 2.
There were fewer deaths in later studies as researchers gained more experience with the procedure. The study evaluated long-term outcomes from HSCT in people with different forms of MS. The transplants took place between andwith a follow-up period of up to 16 years. Several different transplant protocols were followed. Eight deaths 2. Most of these occurred during the early development of the procedure; improvements in patient selection and transplant techniques have significantly reduced the mortality.
Those with the best outcomes tended to be younger, multiple sclerosis research paper, had relapsing MS, multiple sclerosis research paper, lower accumulation of disability and had used fewer MS therapies prior to the transplant procedure.
Additional research is needed to better understand who might benefit from this procedure and how it compares to the benefits of powerful immune-modulating therapies now available, multiple sclerosis research paper.
It tested HSCT in 24 people with MS and active relapsing-remitting disease that was not controlled by disease-modifying medications. Results suggest that after five years, Most of these occurred within the first 30 days after transplant and were related to low white blood cell counts and infections.
This trial, which was funded by the National Institutes of Health, is multiple sclerosis research paper important addition to research needed to determine whether this approach to stem cell transplantation is safe and effective in people with MS. A larger, phase 3 study is in the process of launching see"Ongoing Research in HSCT" above. One participant died and another required intensive hospital care for liver complications. All participants developed fevers, which were frequently associated with infections, and other toxicities.
Read more about this study. Stem cells: Go to a Glossary of Terms Read more about stem cell clinics and questions to ask Read about National MS Society Research in Stem Cells Read a MOMENTUM article about stem cell therapy Multiple sclerosis research paper about treatments available for people with MS Read more about stem cells from the International Society for Stem Cell Research.
Positive Results Announced from Small Clinical Trial of Cell Therapy in Progressive MS March 31, Multiple sclerosis research paper from Italy Report Long-Term Outcomes from Bone Marrow Transplants aHSCT to Treat MS January 20, Study Shows that Stem Cells Derived from Skin Cells of People with MS Make Normal Nerve-Insulating Myelin December 4, National MS Society Releases Recommendations for aHSCT-Bone Marrow Transplant for MS November 11, HSCT Shows Benefits in Some People with Secondary Progressive MS in Small Study February 10, Its Identification Number EIN is 13 - We use cookies to provide an enhanced experience, to keep our site safe and to deliver specific messaging, multiple sclerosis research paper.
By accepting, you consent to the use of all cookies and by declining, only essential cookies will be used to make our website work. More details can be found in our Privacy Policy.
Multiple Sclerosis Research at Johns Hopkins
, time: 2:17CBD Oil and MS: Is Cannabis Oil a Miracle for Multiple Sclerosis? - CFAH
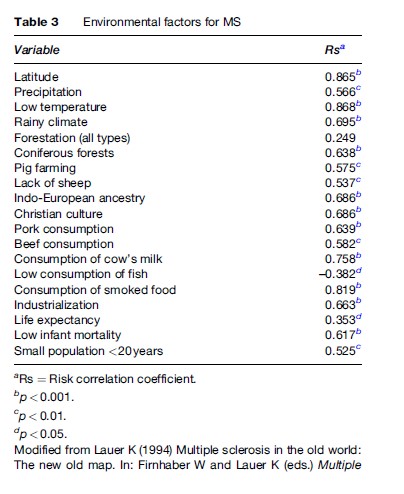
In , some research caused concern that hepatitis B vaccination might be linked with multiple sclerosis (MS), a progressive nerve disease. Numerous studies have evaluated a possible relationship between hepatitis B vaccination and MS The Multiple Sclerosis Task Force, led by Kirsten Potter, PT, DPT, MS, NCS, began its work in fall of The committee's recommendations on the use of measures for individuals with MS were presented at CSM A total of 63 measures were reviewed and recommendations for the use of each in clinical practice, entry-level education, and Aug 22, · Multiple Sclerosis is an area of ever expanding research and escalating publications. Multiple Sclerosis and Related Disorders is a wide ranging international journal supported by key researchers from all neuroscience domains that focus on MS and associated disease of the central nervous system
No comments:
Post a Comment